- Get link
- X
- Other Apps

Every year, it is believed that humanity is increasing climate change and global warming, emitting about 30 billion tons of carbon dioxide (CO2) into the atmosphere of the planet. Scientists from the University of Toronto believe that they have found a way to convert these emissions into energy-rich fuels. In this process can be used widely distributed material in nature - silicon. It should be noted that the silicon contained in the sand is the seventh among the most common chemical elements in the Universe and the second most abundant in the Earth's crust.
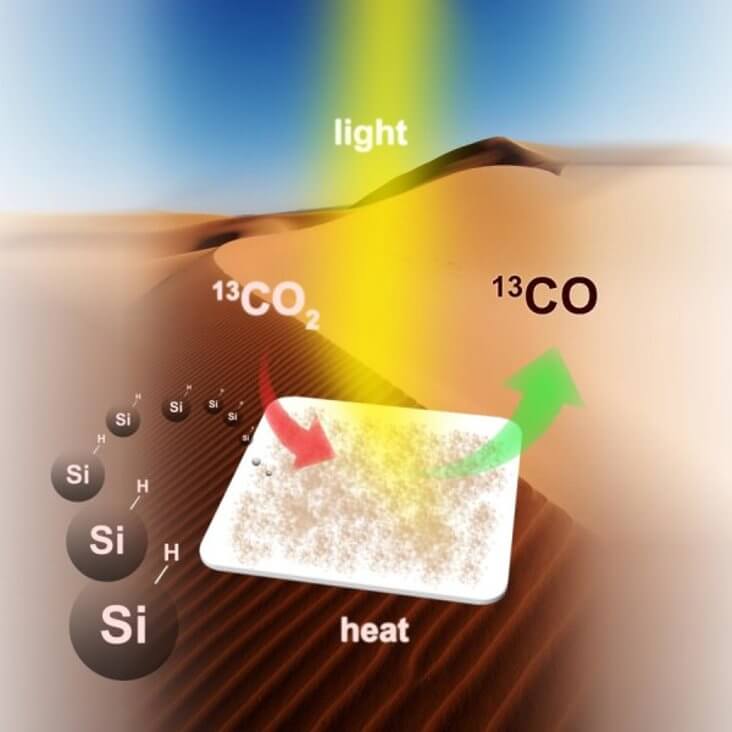
Chenxi Qian illustration showing the conversion of carbon dioxide emissions into nature into energy-rich fuels using nanosilicon (Si)
The idea of converting carbon dioxide into an energy source is not new - for decades, scientists have been searching for materials that could effectively convert sunlight, CO2, water and hydrogen into fuel. The chemical stability of carbon dioxide makes it very difficult to find a practical solution to this problem. It was previously reported that an “artificial sheet” could be used to produce fuel from carbon dioxide.
Professor of chemistry at the University of Toronto, Jeffrey Ozin (Geoffrey Ozin) notes:
A chemical solution to [climate change problems] requires a material that is a very active and selective catalyst that allows the conversion of carbon dioxide to fuel. It is also necessary to make sure that [this solution] is characterized by low costs, is not toxic, and [being ready] for practical use.
In a paper published on August 23, 2016 by Nature Communication magazine, Professor Ozin and his colleagues report silicon nanocrystals that meet all these criteria. Silicon-hydrogen nanocrystals — nanostructured hydrides — have an average diameter of 3.5 nanometers, while possessing sufficient surface area and optical absorption capacity to effectively “harvest” the near-infrared, visible and ultraviolet sunlight waves. In combination with powerful chemical reducing agents on the surface, they effectively and selectively convert carbon dioxide (carbon dioxide) to carbon monoxide.
Potentially, an energy source can be obtained without harmful emissions in the process of its production. Thus, the use of the properties of nanostructured hydrides, as explained by Professor Ozin, can be a commercially interesting method of obtaining fuel directly from sunlight. The goal of the researchers is to show the work of this method in the laboratory and, if successful, to launch a pilot project for obtaining energy using it.
Which of the technologies proposed by scientists for obtaining fuel from carbon dioxide seems to be the most promising?
Based on sciencedaily.com
The article is based on materials .
- Get link
- X
- Other Apps
Comments
Post a Comment